Elliott P. Barnhart1,2,3, Marcella A. McClure1, Kiki Johnson1, Sean Cleveland1, Kristopher A. Hunt2,4 & Matthew W. Fields1,2,5,6,7
1Department of Microbiology and Immunology, Montana State University, Bozeman, MT. 2Center for Biofilm Engineering, Montana State University, Bozeman, MT. 3US Geological Survey, Helena, MT. 4Department of Chemical and Biological Engineering, Montana State University, Bozeman, MT. 5Energy Research Institute, Montana State University, Bozeman, MT, 6ENIGMA (http://enigma.lbl.gov/). 7National Center for Genome Resources, Santa Fe, NM. Correspondence and requests for materials should be addressed to M.W.F. (email: matthew.fields@biofilm.montana.edu)
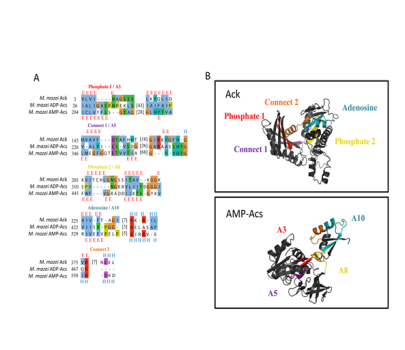
The simplest alkane, one of the most abundant organic compounds on Earth, and the main component of natural gas, methane, has been a byproduct of microbial metabolism for over 3.46 billion years. Methane production, carried out by methanogenic Archaea (methanogens), is an essential part of the global carbon cycle, and acetate, a major microbial intermediate in anaerobic environments, can account for approximately two-thirds of the methane produced on Earth. The ability to utilize and/or excrete acetate to balance carbon and electron flow is widespread across all of life, and the “acetate switch” refers to a metabolic capacity for cells to either excrete or dissimilate acetate-based upon the availability of nutrients. The methanogenic genus, Methanosarcina, is the only identified lineage to have all three mechanisms of acetate utilization. We identified ATPase motif conservation and resulting structural features in AMP- and ADP-acetyl-CoA synthetase proteins that expand the ASKHA superfamily to include acetyl-CoA synthetase. Additional phylogenetic analysis showed that Pta and MaeB sequences had a common ancestor and that the Pta lineage within the halophile archaea was an ancestral lineage. These observations provide a possible scenario for the transfer of pta to an ancient halophilic methanogen that possessed ack and the establishment of the acetate switch pathway. The presented results coincide with the evolution of the first protein kinase as Ack that was previously postulated to be the urkinase and the most ancient protein in the large ASKHA (acetate and sugar kinases/Hsc70/actin) superfamily of phosphotransferases that share an ATPase domain through gene duplication and divergence. The presented evolutionary relationship between the genes provides an evolutionary path for the development of the acetate switch that was then shared between Archaea and Bacteria. The extensive distribution of the acetate switch throughout Bacteria and Eukarya suggests significant contributions to the evolutionary fitness of many organisms across all domains of life. Recent work indicated that a brief period of the Archaeon eon coincided with the rapid diversification of bacterial lineages that gave rise to 27% of modern gene families involved in cellular metabolism. The evolution of the acetate switch and the development of the ASKHA superfamily may have contributed to this early metabolic expansion in biology but may have also contributed to mass extinctions via large biogenic methane releases.
