 Congratulations to Joshua Jiaqi Huang, a UC Berkeley graduate student researcher in Biosciences senior faculty scientist Adam Arkin’s group, who represented ENIGMA and Biosciences at the 2024 BioEPIC Research SLAM.
Congratulations to Joshua Jiaqi Huang, a UC Berkeley graduate student researcher in Biosciences senior faculty scientist Adam Arkin’s group, who represented ENIGMA and Biosciences at the 2024 BioEPIC Research SLAM.
Huang presented his work studying how Cupriavidus necator, a bacteria found at ENIGMA’s highly contaminated Oak Ridge Reservation field site, controls the storage of carbon inside its body, and how that capability could be harnessed to produce sustainable bioplastics. Huang’s project is one of ENIGMA’s many multi-scale research projects aimed at understanding how microbial communities in the water and surrounding sediment in the field adapt to their environment and function to cycle nitrate and uranium.
ENIGMA’s goal of building mechanistic and predictive models for how microbes affect the subsurface environment is just one piece of the BioEPIC puzzle: the other science focus areas that will be housed in BioEPIC will each work at different scales to uncover how microbes impact Earth’s ecosystems, from soil and plants to watersheds.
Explore Huang’s research in his own Words:
In the same way humans save money in case of financial hardship, bacteria also save resources for tough times. I’m investigating how the bacteria C. necator manages bioplastic carbon storage under different carbon and nitrogen conditions. The bioplastic polyhydroxybutyrate (PHB), an important tool for C. necator survival; is a significant route of carbon sequestration in the ecosystem, and a decomposable material for industrial uses. As such, understanding the strategy of how C. necator makes the bioplastic PHB could help with both understanding the carbon cycle and improving the efficiency of biomanufacturing.
Similar to humans, even genetically-similar bacteria can have huge variations in their individual behavior. To resolve patterns within this colossal variation and noise, we needed a way to observe large numbers of individual bacteria over a long time period.
To achieve that, we built a microfluidic system that traps bacteria into tiny channels orthogonal to the nutrient flow to keep bacteria in constant conditions under a microscope. With this device, we can track up to 100,000 bacteria at single-cell resolution for up to five days. By reducing nitrogen concentrations down to 10^-12 grams per liter, we found that contrary to common belief, C. necator does not accelerate its PHB production in low nitrogen concentration. At exceedingly low nitrogen concentration near the top of the channel, cells stop growing while they keep accumulating PHB, which increases the PHB yields by improving PHB mass per unit biomass (but not the production rate). We believe that is what the others previously observed in bulk experiments.
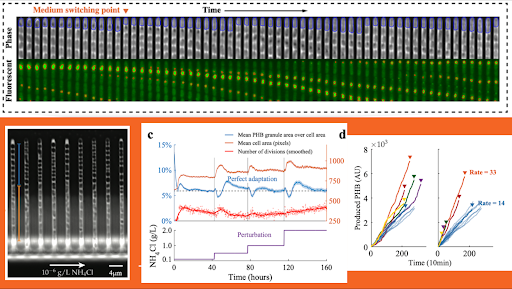
C. necator actively maintains homeostasis over an extensive range of nitrogen concentrations; achieving a constant PHB production rate even when nitrogen increases by several orders of magnitude. To improve production rates in an industrial setting, we will need to overcome this tightly-controlled homeostasis system.
A very small number, about 2%, of the cells produce PHB at about twice the rate of the others. We believe this is a bet-hedging strategy to give the population better resilience to extended periods of famine with a few of them surviving significantly longer than the others. The strategies we discovered help C. necator survive in the wild, and sequester carbon from the atmosphere. The manipulation of these strategies can also help us produce more PHB towards a more decomposable future.
