Diverse bacteria and archaea rely on unusual methionine synthases.
The Science
Methionine is one of the amino acids that make up proteins, and the final step in methionine synthesis is the transfer of a methyl group. Usually, the methyl group is obtained from methyl folates, but some anaerobic microbes use vitamin B12-binding proteins instead. By analyzing the genomes of diverse bacteria and archaea, we identified four families of folate-independent methionine synthases. Three of these families co-occur with B12-binding proteins, which indicates their likely partners. The fourth family does not require vitamin B12; instead, it obtains methyl groups from an oxygen-dependent partner protein.
The Impact
For many microbes, we know little about them besides their genome sequences. To predict what these organisms are capable of, scientists check to see which gene families their genomes encode. The four families of folate-independent methionine synthases help to explain how many different kinds of bacteria and archaea can grow without added nutrients. We also discovered a new type of enzyme – an oxygen-dependent methyltransferase – which might be useful for chemical engineering.
Summary
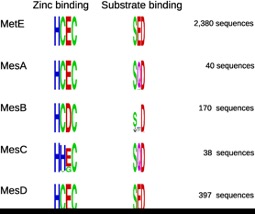
While working on the GapMind tool for annotating amino acid biosynthesis, we noticed that diverse bacteria and archaea do not have any of the known forms of methionine synthase. Many of these organisms can grow in the absence of methionine, which shows that they do have methionine synthases. By analyzing their genomes, we identified four families of “core” methionine synthases that lack folate-binding domains. Two of these families corresponded to proteins that had been studied before, and two were novel. The novel families (MesC and MesD) have similar catalytic and substrate-binding features as other methionine synthases (see figure). Genetic data confirm that MesD is a methionine synthase. But unlike the other folate-independent methionine synthases, MesD does not rely on vitamin B12 – instead, it relies on an oxygen-dependent partner protein.
Contact
Morgan Price
Lawrence Berkeley National Laboratory
Publications
Price, M.N., Deutschbauer, A.M. and Arkin, A.P. “Four families of folate-independent methionine synthases.” PLoS Genetics (2021). [10.1371/journal.pgen.1009342]
Related Links
GapMind: Automated annotation of Amino acid biosynthesis, Arkin Group, Lawrence Berkeley National Laboratory.
