Vuono D.C., Read R.W., Hemp J., Sullivan B.W., Arnone, J.A., Neveux I., Blank R.R., Loney E., Miceli D., Winkler M-K.H., Chakraborty R., Stahl D.A., Grzymski J.J. 2019. Resource Concentration Modulates the Fate of Dissimilated Nitrogen in a Dual-Pathway Actinobacterium. Frontiers in Microbiology Vol 10, 3 [doi]:3389/fmicb.2019.00003{PMID}:30723459 PMCID:PMC6349771 OSTI:1560586
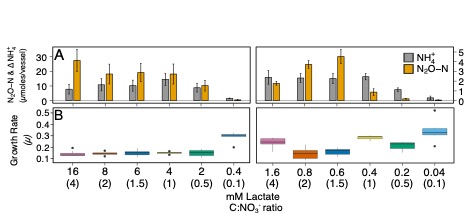
In this study, we tested the effects of C:NO3− ratio and substrate concentration on pathway selection between ammonification and denitrification in the dual-pathway organism Intrasporangium calvum C5. We challenge the paradigm that C:NO3− ratio controls pathway selection based on a simple principle: ratios do not account for substrate concentrations, which can impose resource limitation for C or NO3−
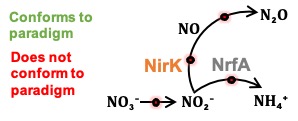
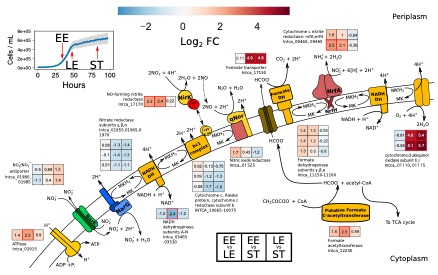
Respiratory ammonification and denitrification are two evolutionarily unrelated dissimilatory nitrogen (N) processes central to the global N cycle, the activity of which is thought to be controlled by carbon (C) to nitrate (NO3−) ratio. Here we find that Intrasporangium calvum C5, a novel dual-pathway denitrifier/respiratory ammonifier, disproportionately utilizes ammonification rather than denitrification when grown under low C concentrations, even at low C:NO3− ratios. This finding is in conflict with the paradigm that high C:NO3− ratios promote ammonification and low C:NO3− ratios promote denitrification. We find that the protein atomic composition for denitrification modules (NirK) are significantly cost minimized for C and N compared to ammonification modules (NrfA), indicating that limitation for C and N is a major evolutionary selective pressure imprinted in the architecture of these proteins. The evolutionary precedent for these findings suggests ecological significance as evidenced by higher growth rates when I. calvum grows predominantly using its ammonification pathway and by assimilating its end-product (ammonium) for growth under ammonium-free conditions. Genomic analysis of I. calvum further revealed a versatile ecophysiology to cope with nutrient stress and redox conditions. Metabolite and transcriptional profiles during growth indicate that enzyme modules, NrfAH and NirK, are not constitutively expressed but rather induced by nitrite production via NarG. Mechanistically, our results suggest that pathway selection is driven by intracellular redox potential (redox poise), which may be lowered when resource concentrations are low, thereby decreasing catalytic activity of upstream electron transport steps (i.e., the bc1 complex) needed for denitrification enzymes. Our work advances our understanding of the biogeochemical flexibility of N-cycling organisms, pathway evolution, and ecological food-webs.